Telomeres: everything you always wanted to know
Back in 2020 when I wrote the Longevity FAQ I had a section on telomeres and some cursory examination of how much they might matter for aging. Now that I know more it's time to revisit it, including also some historical notes and some papers that came out after I wrote that FAQ.
The direct reason for writing this post is that often when I say that I work on aging (at Retro Biosciences, we are hiring!) I am asked if "that's the telomeres thing". For some reason, telomeres became tied to aging broadly a few years ago and I wanted to understand why while at the same time I wanted to understand how relevant they actually are, as I suspect myself and others have overcorrected much away from "telomeres are the key to aging" in the direction of "they don't matter that much". From time to time one can see things from seemingly reputable outlets like The Nobel Prize twitter account saying that "the secret [to why we age] lies in our telomeres" and people in the field making fun of them.
To start, telomeres are the regions at the end of chromosomes, so all of our cells have telomeres. The details of what these regions look like are covered in a bit more of length in the FAQ; for this post what matters is the fact that when a cell divides it has to copy its DNA. When this happen, telomeres shrink. When the telomeres are too short the cells stops replicating and becomes 'senescent'. There is an enzyme called telomerase. Telomerase is not a single protein, rather it's a complex made of (in humans) proteins TERT (telomerase reverse transcriptase), TERC (telomerase RNA), and DKC1 (dyskerin).
It's important to note, as this gets lost in discussion sometimes, that when someone talks about telomeres shortening or lengthening it's usually the telomeres of white blood cells like T-cells, because accessing blood samples is the easiest. Changes in the telomeres of those cells does not imply changes in telomeres elsewhere. T-cells in particular can divide and the populations of different kinds of white blood cells can change with external factors, so it can also be the case that telomeres as usually measured lengthen simply because cells with shorter telomeres die, so average telomere size then increases.
For example, a person more exposed to infections and carcinogens may make their T-cells work harder, implying more divisions and telomere shortening. That person might die earlier and in a cross-sectional study we may see that shorter telomeres correlate with earlier mortality but this will just be a downstream biomarker of exposure to disease in this case, and not a direct causal driver.
What cells can divide (and so which cells have telomeres that can potentially shorten?) fibroblasts, endothelial cells, smooth muscle cells, glial cells, or astrocytes. In contrast, neurons or myocytes do not divide so we shouldn't expect telomere shortening to have much to do with the aging of those cells.
The usual function ascribed to telomeres is as an anti-cancer mechanism: if we cell begins dividing too much then its telomeres will progressively shorten and it will stop dividing (or die). To overcome this, cancers end up reactivating telomerase to keep their telomere length. Absent telomerase they can also engage in ALT (Alternative Lengthening of Telomeres), so all else equal we would expect telomere elongation to lead to higher likelihood of cancer, but this is a simplistic view, as longer telomeres might also mean a better functioning immune system (T-cells that can divide more) and less inflammation (fewer senescent cells).
The history of telomeres and their relevance goes back to at least Leonard Hayflick's 1961 and 1965 papers on what he called senescence: in culture, cells eventually stop dividing; in that paper he pointed to factors intrinsic to the cells but at the time he did not link that to telomeres. Cells extracted from older donors would stop dividing earlier, pointing to the existence of a limit to the total amount of divisions a cell could undergo, and that clock starting ticking since we are born, not just in vitro.
It wasn't until 1970 when a Soviet scientist, Alexey Olovnikov had a sudden realization:
The Theory of Marginotomy [JR: Telomere shortening] came to me in that Moscow subway station. I heard the deep roar of an approaching train coming out from the tunnel into the station itself. I imagined the DNA polymerase to be the train moving along the tunnel that I imagined to be the DNA molecule. I thought that this polymerase cannot begin to copy from the very beginning because there is a dead zone between the front end of the polymerase molecule and its catalytic center. This is analogous to the dead zone between the front end of a subway car standing at the beginning of the subway platform and the nearest entrance door to the first car. After this serendipitous underground brainstorm, which happened in the Fall of 1966, I wrote to Hayflick to ask some questions about his discovery and he sent additional unpublished data to me. I then spent several years thinking about this idea before publishing it in the central journal of our Academy of Sciences, Doklady Academii Nauk SSSR
So he was the first person to propose that it is telomere shortening due to imperfect DNA replication that is causing what Hayflick had observed.
Meanwhile in the US, Elizabeth Blackburn, who would later win the Nobel Prize for her work on telomeres publishes in 1978 the sequence of these regions in the chromosomes of Tetrahymena thermophila, a protozoan. Telomeres turns out to be TTAGGG (repeated 3000 times or so) in humans whereas in tetrahymena they turn out to be TTGGGG. Other species have other repeats. She would be working with Jack Szostak over the following years on understanding telomeres.
Later, in 1984 one of Blackburn's students, Carol Greider discovers the enzyme telomerase, initially called the telomere terminal transferase.
Blackburn and Greider's work also noted that Tetrahymena with knocked out telomerase became sick and died quickly (1990), so telomeres and telomerase seemed key to keeping cells alive. Unlike Hayflick's work, which was in cells from humans, Tetrahymena are complete unicellular organisms, so their work provided more robust evidence that telomeres could be driving senescence at the organismal scale. They also noted that this system may have evolved to prevent cancer, putting senescence and cancer at odds. But in principle, one could think, if there was a way to prevent cancer, would telomere restoration prevent aging at the organismal level in more complex organisms like mice?
Intrigued by these findings, Geron Corporation launched in 1990 with Greider and Hayflick as advisors looking to develop telomere-based therapies for aging. It was seemingly Geron and its PR department that started the "telomeres drive aging" meme in the general population, according to Greider.
In the 90s a book titled Reversing Human Aging came out, also supporting telomere shortening as key explanation for aging. In that book, we can find telomeres referred to as the "clocks of aging" and that "Aging is caused by aging cells and aging cells by aging telomeres."
In 1998 for the first time it becomes possible, thanks to Geron scientists to take regular cells and turn on telomerase permanently preserving telomeres. This enables the cells to escape senescence. The process was dubbed 'immortalization'. In the media we get quotes like "We found that biological aging can be put on hold"
Now at this point it would make sense to take this and put it in mice to see what happens. So in 2001, the Blasco lab turned on telomerase in a certain type of cell in the skin of mice. The result wasn't longer lived mice, but rather they mice got more skin cancer (and faster wound healing), confirming the double-edged sword nature of telomeres.
A year after another paper comes out studying the effect of turning on telomerase in the entire mouse since birth. The results confirm Blasco's: they also saw an increase in cancers.
Looking at these two papers and a few others, 2004 supposes an inflection point for the telomere hypothesis of aging. Gerontologist João Pedro de Magalhaes publishes Telomeres and Telomerase: A Modern Fountain of Youth? where he concludes in very skeptical terms that:
though telomerase may be used in regenerative medicine and to treat specific diseases, it is unlikely to become a source of anti-ageing therapies.
One key point made there that Greider also made trying to counter the telomere hype is that there's no correlation between telomere length and the longevity of a species. Mice have longer telomeres than we do but they live much shorter lives. A conclusion is drawn there that
Therefore, telomere length and/or telomerase activity do not explain why humans age slower than other primates and mice
But this is not quite right. Many years after (in 2019) that Blasco and others would show that while it is correct that telomere lenght does not correlate with longevity, the rate of shortening (which is different in different species) does correlate fairly well:
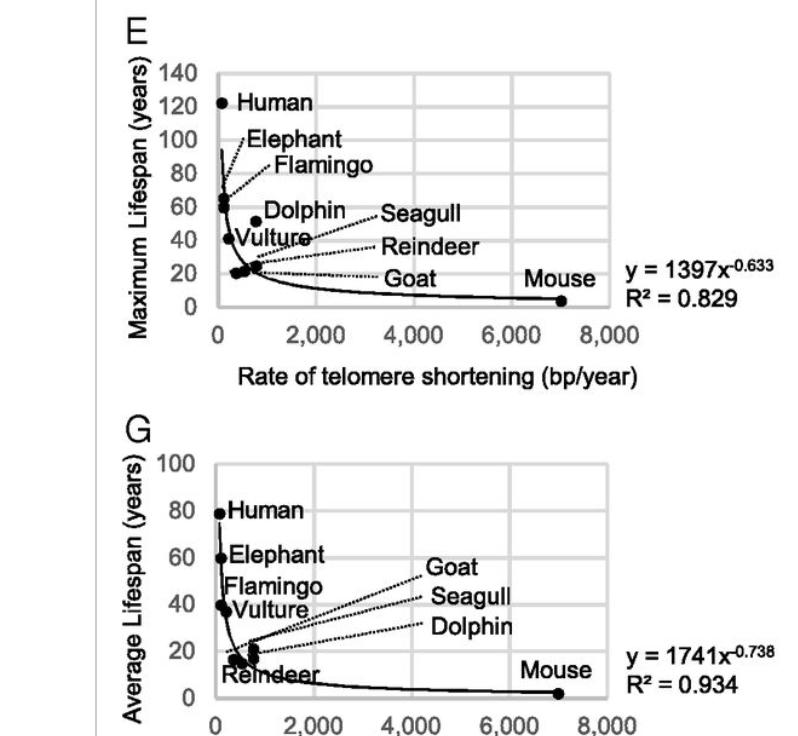
This doesn't mean that they set the pace of aging! Other things like the rate of accumulation of DNA mutations also correlate well with lifespan. In general if many mechanisms are necessary for long life, then evolution will jointly optimize them all for progressively increased lifespans. There's no living long with fast shortening telomeres OR high mutational burdens it seems.
After the de Magalhaes critique, Blasco publishes in 2008 for the first time a report of lifespan extension using telomerase overexpression albeit in cancer-resistant mice. Fine, one could say, keeping telomeres long causes cancer, but what if we avoid that by genetically engineering the mice to be more resilient than normal mice will ever be, do we get healthier longer lives in mice? The answer is yes we do. Compared to the baseline non-telomerase'd cancer resistant mice, the ones that have constant telomerase expression:
- Preserve skin thickness with age
- Preserve villi structure in the GI tract (and associated gut barrier integrity->less leaky gut)
- Do better in a motor test
- Show improved glucose metabolism
- Live substantially more, and more mice reach very old age (42% vs 8%)
- As one might expect, telomeres are longer
Promising!
Understanding telomere biology turned out to be important enough to earn Blackburn, Greider, and Szostak a Nobel Prize in 2009. (Sad day for Olovnikov)
Then one year after the telomere hypothesis continues to receive support with the publication in 2010 of Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Note that these are not regular mice, but mice that do not have telomerase at all; their telomeres are much shorter than usual.
With this paper, there was then evidence than longer telomeres could, in a context (cancer-resistant mice) extend lifespan as well as telomerase deficiency could shorten it and that adding back telomerase could reverse that. But could that work in regular mice?
In 2012, the Blasco lab once again published a paper where they take regular mice and using an AAV gene therapy cause widespread telomerase activation. The result: increased lifespan regardless of whether the therapy was delivered early in life (24% increase) or later in life (13% increase). This increase was accompanied by other health benefits. Importantly, they got less, not more cancer. They especulate that because the AAV would get diluted in fast dividing cells, cancer cells would not be able to benefit from this additional telomerase.
The same year, a consortium of aging researchers published what's probably the most cited paper in the field, Hallmarks of Aging, where they establish the importance of telomeres as a consensus view.
Then later, in 2015 we get an interesting case of self-experimentation. Elizabeth Parrish, the CEO of a biotech company called BioViva injects herself with a gene therapy to lengthen her telomeres. They'd do it again in 2021 in some volunteers somewhat quietly, I hadn't heard of this study until today!
That same year we get another critique of the involvement of telomeres in aging. The paper starts by pointing out that knocking out telomerase in mice and breeding those mice, which leads to decreasing telomere size in the newborns over the generations does not decrease lifespan in the first generation (but it does later), implying that telomere length in mice is not a limiting factor for their lifespan. What then about the 2012 paper from the Blasco lab using AAV? The author argues the sample size was small and perhaps telomerase expression itself (and not just telomere length) is behind the effects seen. He also expresses doubt that telomeres can be involved in aging because mice telomere length varies a lot between inbred strains vs true wild mice without correlating to lifespan (Something we have thing is no mystery: what matters is the shortening rate, but that Blasco paper hadn't come out yet).
Blasco would provide an answer to one of these critiques in an impressive 2019 paper: mice with hyperlong telomeres live longer (12.8%) and are healthier in various ways. These mice were bred to have longer telomeres without overexpressing telomerase so one can rule out the potential action of telomerase in having the observed effects. They also get less cancer, suggesting that the anti-cancer effects from a better immune system or the inflammation reduction from longer telomeres outweigh the tumorigenic risks of telomerase.
So after the AAV paper from 2012, one'd think that many in the field would be relatively bullish on telomeres, but not quite. Aubrey de Grey, in 2018 was asked about the promise of telomere lengthening, said that he was worried about the risk of cancer, pointing to some of the studies showing an increase in cancer. To explain the results where mice with telomerase overexpression living longer, he points to the observation periods being too short (definately not the case for the AAV paper) or the animals being particularly cancer resistant (the case with the earlier papers).
Lastly, also cited by de Grey are some studies of human genetics using mendelian randomization to see what happens when the rate of telomere shortening is altered in humans. The result is a slight increase in cancer, a slight decrease in coronary artery disease, and a null effect on lifespan, implying that for humans in particular telomere length wouldn't extend life. Perhaps! But remember that in mice the length of telomeres doesn't determine lifespan in the general population despite the fact that hyper-long telomere mice do show improved lifespan. Could it be that people with hyper-long telomeres live longer? I don't expect so, given how slow human telomeres shrink, but the possibility exists. There are other papers one could point to that purportedly show that long telomeres in humans are deleterious; people with mutations in the POT1 gene have longer telomeres but one might reply that the mutation itself is having deleterious effect beyond telomeres. One could imagine a one-off AAV therapy that lenghtens telomeres and if cells turns cancerous and start dividing, the AAV will be diluted so the effects on cancer will be minimized (as Blasco et al. speculated in their own paper).
Another critique is from the Ehninger lab in 2022. The authors point out, correctly, that there's no correlation between telomere length and lifespan across mice strains, inferring from that that telomere shortening does not underlie murine aging. But given the hyperlong telomeres paper, this doesn't seem right. They also point to telomere lengthening increasing carcinogenesis, but they bunch together various kinds of interventions. Constitutive telomerase activation is not the same as transient, delivered via a virus (which would be closer to a hypothetical clinical translation). Ehninger just counts the number of studies saying there is more vs there is less cancer. 2 studies showing there's less (the AAV study and the hyperlong telomeres paper) vs others using constitutive expression. This is obviously not a fair reading of the evidence!
The latest big and somewhat controversial paper in telomere land is this one from Liz Parrish and colleagues (See Pubpeer for why it is controversial) but I don't think it's controversial enough to discredit the main relevant result: a replication, in a way, of the Blasco AAV paper showing lifespan extension, only that using CMV instead, showing a 41% increase in lifespan.
So what should we expect to see in humans? A disease, dyskeratosis congenita (DC) might give us some clues. Patients with DC have short telomeres and as a result they present with defects in organs and tissues that experience faster turnover: where we would expect telomeres to shorten faster (skin, nails, lungs, bone marrow). So we mighte expect that if we lived long enough and our telomeres shorten sufficiently, we would begin to experience something like this. According to this paper, telomere length (in white blood cells) in DC patients is similar to the telomere length of someone that might be over 90 years old, so it wouldn't be totally surprising if around that age people would start experiencing such symptoms naturally and given that bone marrow is one of those tissues, telomere-shortening ends up being a bottleneck for healthy lifespan extension at advanced ages.
A visual summary of what seems to be the case, at least in mice, would be something like this:
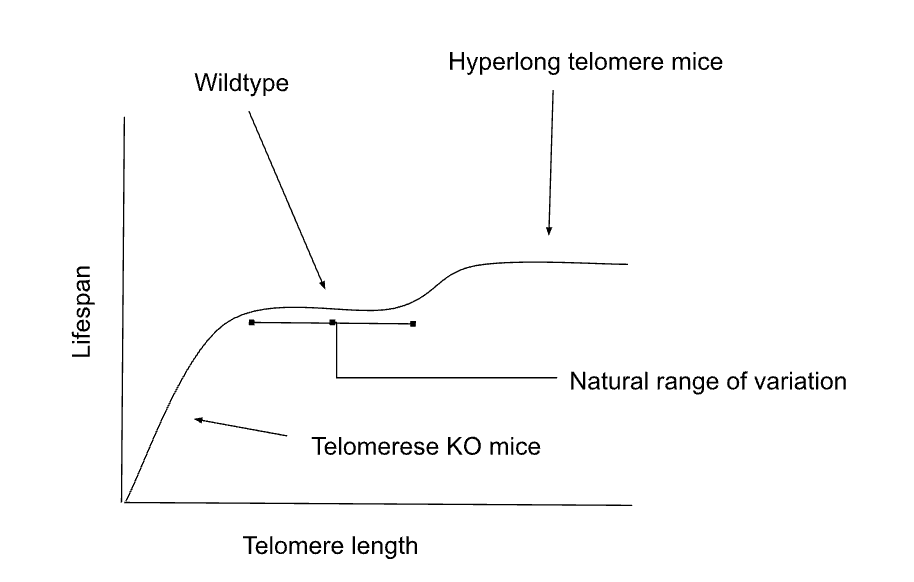
Now to finish, I want to address studies that seem to find that telomere length changes with some lifestyle intervention. The first point to make there is that telomere measurement is an imperfect art. The second is that as I pointed out in the introduction all cells have telomeres and mostly people mean "telomeres in white blood cells" and those can change (seemingly elongate even) just to address an infection. But you could say well ok! Maybe telomeres are more an indicator of damage, so what about measuring them to look at lifespan? I wouldn't bother. If you want to look at things like that, DNAme clocks or just a good old frailty index have far more predictive value for mortality risk. Telomere length tells you very little (unless they are extremely short, but then it'd be because of a rare genetic disease, and not because of anything you have done). This video has a reasonably good explanation of telomere as (poor) biomarkers of aging.
So in conclusion, telomere lengthening via gene therapy, and perhaps in combination with cancer-targeted therapy continues to be worth investigating as an anti-aging intervention, though it may be superseded by ex vivo reprogramming or iPSC-derived therapies, as reprogramming lenghtens telomeres so one gets telomere reset for free along with the epigenetic age reset.
The hype of the early days ("telomeres are the key to aging") is as wrong as the anti-hype ("they lead to cancer, there's no evidence that manipulating telomeres helps with aging").
Citation
In academic work, please cite this essay as:
Ricón, José Luis, “Telomeres: everything you always wanted to know”, Nintil (2023-10-17), available at https://nintil.com/telomeres/.
Comments