Rapamycin is not an aging drug. But what is an aging drug?
Summary
Rapamycin increases lifespan in all wildtype model organisms where it has been tested. It improves many aspects of health in old and young animals and slows down decline, but it also worsens or leaves unaffected a number of them. It does not reverse aging systemically or permanently either at the cellular or organismal level. In comparison to other lines of research like reprogramming, rapamycin and other mTOR-targeting interventions are a local optimum, not part of the roadmap towards robust healthy lifespan extension
The title of this post will spark some controversy. But it is, under a reasonable interpretation, not false, just spicy 🌶️.
It make sense to start from the perhaps less controversial view that "rapamycin is an aging drug" and explain why that is wrong as well.
My issue with those that talk about rapamycin as an "(anti)aging drug", or a "geroprotector" is that it's often unclear what of this is being said
- There's a unified thing called aging that affects everything that goes wrong with chronological time. Rapamycin modulates that one lever and as a result improves health and lifespan.
- Rapamycin increases lifespan and has broad health effects. An aging drug is anything that broadly does this.
(2) is a perfectly valid statement to make. (1) is known to be false. But I think it's easy to fall into the (1) frame of thinking when reading some of the rapamycin papers, or sometimes popular press coverage thereof. So: rapamycin is an aging drug in the sense of (2) and it is not an aging drug in the sense of (1). We should prefer to say that "rapamycin slows some aspects of aging" than "rapamycin slows aging" or "rapamycin slows the aging process".
As I will mention below, rapamycin does not simply turn back or stop the clock. It doesn't take every organ and resets it by some number of years. Rapamycin leaves some functions untouched and makes others worse.
Now at this point some may go "duh of course, aging is multifactorial and rapa is addressing parts of it" whereas other might go "Heresy! Surely the papers that show that rapa doesn't help with everything must be wrong or something". The second is more clearly a caricature. Even well known proponent of rapamycin as an aging drug take it as a given that it has its downsides (the mouth ulcers are well established in humans). But then, if we agree that "rapamycin is just affecting some aspects of aging" then we have to be very careful when reading claims that "rapamycin slows aging" as that sounds like it is slowing all aspects of aging and perhaps even that all aging is mTOR-driven.
I suspect that the intimate relationship between rapamycin and aging in some's conceptual schemes dates back to the origins of the field. The mTOR-IGF axis is where the field was born, studying caloric restriction. CR also has lifespan-increasing and broad health-promoting effects, so the idea that there was a master program regulating lifespan and health and that this is tied to mTOR can be coming from there. This mTOR maximalism view is presented by eg. Blagosklonny but it is not an explicit majority view.
As a brief introduction, rapamycin is an mTOR inhibitor that is able to extend lifespan in the usual model organisms: yeast, flies, worms, and mice. Its lifespan-extending effects are perhaps one of the most consistently replicated findings in the field, showing success in the Interventions Testing Program, a large sample size study using outbred mice of various interventions aiming to extend lifespan.
In worms, yeasts, and flies, rapamycin seems to extend lifespan because of an autophagy-inducing effect (i.e. it depends on the presence of key autophagy-related genes to work) whereas in mice there are additional effects present, possibly anti-inflammatory (Rubinsztein et al., 2011) and surely anti-cancer. In addition to living longer, rapamycin slows down the appearence of various age-related phenotypes in muscle, tendons or liver (Wilkinson et al., 2012).
Given that rapamycin extends lifespan and improves health, how could one possibly say that rapamycin is not an aging drug? Is it not impacting biological aging?
I honestly don't understand this view. Rapamycin increases lifespan and delays declines or improves function in multiple (every?) aged tissues when started in middle age. What is the alternative explanation other than impacting biological aging?
— Matt Kaeberlein (@mkaeberlein) December 4, 2022
First, one has to question the narrative that rapamycin improves "health" as a unified entity. Rapamycin improves some aspects of health and worsens others. The Hallmarks of Aging paper cites the Wilkinson paper from earlier to say that "rapamycin delays multiple aspects of aging in mice". However it completely fails to mention that the paper also says that rapamycin accelerates the appearence of cataracts and leads to testicular degeneration. In humans, mouth ulcers are a common observation as well (Peterson et al., 2016). It's up to each individual to judge if the traeoffs will be worth to them, given other interventions they may be undergoing.
In my view the most rigorous and comprehensive examination of the effects of rapamycin in mice has been done by Dan Ehninger in these papers:
- Deep phenotyping and lifetime trajectories reveal limited effects of longevity regulators on the aging process in C57Bl/6J mice (2022)
- Longevity, aging, and rapamycin (Ehninger et al., 2014)
- Rapamycin extends murine lifespan but has limited effects on aging (Neff et al. 2013)
The overall point made by Ehninger in these papers is that rapamycin is extending lifespan in mice in part via cancer suppression, and that in addition to that, it is having effects on only a subset of age-related phenotypes and furthermore that it does also improve function in young animals so he claims that (his words) there is currently little evidence available to support the notion that mTOR inhibitors slow the rate of mammalian aging. Ehninger acknowledges that the lifespan-extending effects of rapa are not just due to the anti-cancer effect: cancer is not a cause of death in yeast, flies, or worms, where lifespan effects are also seen. But it may be that the reason that rapa increases lifespan in those is different from the reason it increases lifespan in mice.
To make this more clear: To extend lifespan in mice one must ultimately slow down cancer because most mice die of that, which mTOR inhibition does either indirectly (inflammation reduction, for example) or directly (slowing down growth of the cancer itself). Then there's a separate autophagy-enhancing effect which may be doing little for the mice in terms of mortality, but a lot for the lower organisms.
Importantly, it is possible to extend lifespan in a given organism in many ways. Whether an intervention is addressing aging depends on the definition you are using. That is, a definition that puts a heavy weight on "lifespan extension" will classify chemotherapy and statins as a aging drugs in that they address phenotypes that are more common with age (cancer and CVD respectively) and they extend lifespan as a result.
Ehninger points out that if a drug is having the same effect in young than it does in old, it is not really addressing aging but rather reversing age-related phenotypes via aging-independent mechanisms.
Take muscle mass; after development it declines. Reducing the activity of myostatin can increase muscle mass, and this increase can happen in both young and old. This treatment could plausibly reverse sarcopenia in the old (ie shifting muscle mass % closer to young levels).
Now one might say: A myostatin knockdown is not really an aging intervention because it's addressing one disease directly (sarcopenia) through a very proximate regulator of muscle mass. It is not systemic. To which one could say: ah but did you know follistatin overexpression (which reduces myostatin signalling) perhaps increases lifespan (Kumar et al., 2022). Perhaps follistatin treatments are more systemic than one thought at first! Perhaps it's acting through cell-to-cell signalling which is a hallmark of aging. Given this, is follistatin as much of an anti-aging therapy as rapamycin is? Again, depends on how you define aging. I personally think one should not obsess over this question, but rather about what works and what doesn't to improve healthy lifespan in a sustained way.
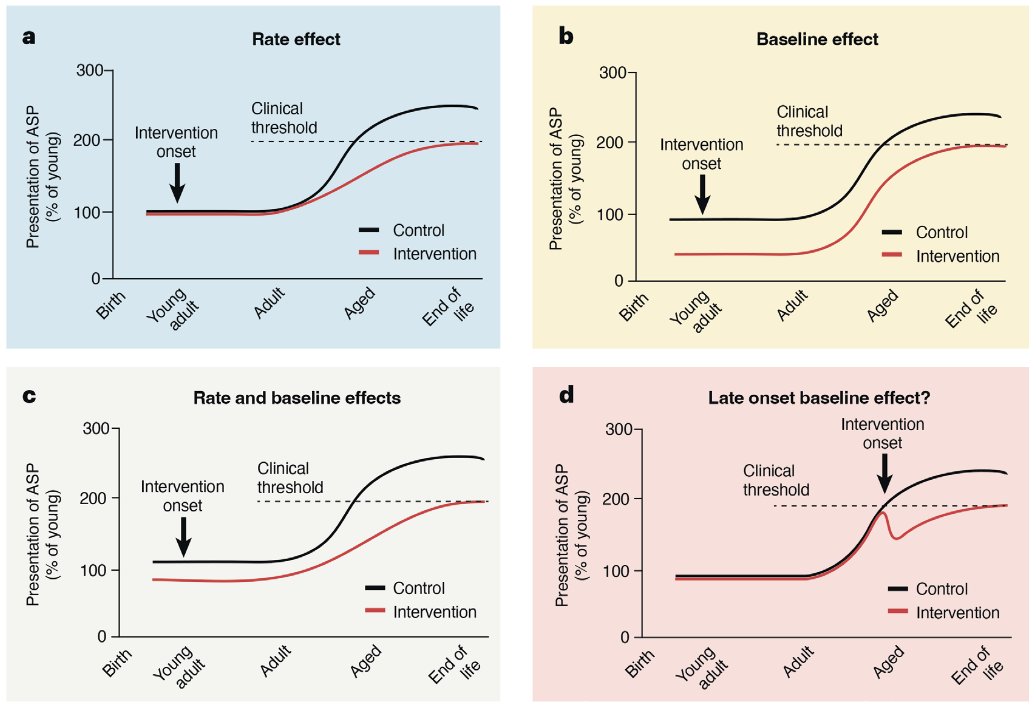
Rapamycin connoiseurs will say that in the latter paper (Neff), the rapa dose was lower than what has been used elsewhere (Johnson et al., 2015) and that this might by why the effects are not as systemic. Even with this, we still have the negative effects found elsewhere and found in humans sometimes like mouth ulcers. Ehninger uses in the later 2022 paper an mTOR deficient mouse, where mice constitutively produce less mTOR. This model recapitulates known effects of rapamycin: lifespan extension (~20%) coupled with broad health effects (Wu et al. 2022). The Ehninger paper measures 208 phenotypes out of which 117 change with age (age-sensitive phenotypes, ASP). They then classify the effects according to whether they have an aging interaction (ie they show an effect in aged but not young animals) or only a genotype interaction (if the effect is present in both aged and young of the same genotype). The result here:
- 16% of ASPs are improved in old but not young ("true effect on aging" in Ehninger's view)
- 31% of ASPs are improved in both old and young ("health-promoting, aging-independent effect" in Ehninger's view)
- 36% of ASPs are unchanged by the mTOR-deficient genotype
- 13% of ASPs were made worse by the mTOR-deficient genotype
While one can quibble with the exact phenotypes they are measuring and whether a number is 15 or 10%, given other papers, it does seem a robust finding to me from other papers: put it simply rapamycin extends lifespan and improves some aspects of healthspan while worsening some others.
Reprogramming as only current approach to systemic, permanent, rejuvenation
Ultimately rapamycin is a small molecule drug. What it can do is limited: It can bind FKBP12 and jam mTOR and that's it. That in turn turns up autophagy and other cellular programs that contribute to general cellular health, leading to increases in lifespan and healthspan. But it also reduces the rate of protein synthesis and has other negative effects. Rapamycin is not an endogenous metabolite that goes down with age (the case of taurine), but rather a hack to crank up pathways that have effects that we mostly want. However, the effects of rapamycin are not permanent nor they reverse aging at the cellular level, rather they slow some aspects of aging down (Kabacik et al. 2022; Horvath et al. 2019). We should think of rapamycin mostly as a way to slow down aspects of aging transiently, but not as a path towards robust age reversal. A "better rapamycin" in my view, is not particularly worth seeking, compared to alternatives.
Note that this is an empirical observation: It could be the case that an intervention that say boosts autophagy lets the cell "catch up" on garbage collection, resetting the age of the cell permanently. But in practice, this is not what rapamycin seems to be doing. It stops and delays, not repairs and reverses.
The only intervention that can permanently and systemically reverse aging at a cellular level (with minor exceptions like DNA mutations) that we know of as of today is reprogramming. I'd like to be wrong on this one: more interventions are better so if you have something in mind that can reverse aging permanently, send it my way at [email protected] .
Unlike therapies targeting various aging hallmarks, reprogramming is permanent. Once a cell has been made into an iPSC, it stays young in almost every possible way, and once the cell is made back to its type of origin, the clock is reset, without the original OSKM factors having to be present anymore. Under a very strict definition where "age reversal" means that every measurable thing has been reverted back to a young state, the irreversibility by reprogramming of DNA mutations would disqualify it. But under a more relaxed "general reversal" definition, it is second to none.
The issue with reprogramming (or partial reprogramming) is that it's harder to control in vivo than rapamycin so applying the intervention is not as easy as giving some mice an Eudragit-coated serving of rapa. It's going to take some engineering to make it work there. Alternatively, one can reprogram ex vivo cell types one at a time and then engraft them back, fully rejuvenated cells.
But aren't the hallmarks of aging connected? Yes to some extent but not fully: per the Kabacik paper earlier rapamycin indeed affects others. But we already know the deterioraton of epigenetic information in cells is something rapamycin does not reverse. Within the hallmarks, some are more upstream than others, and that which rapamycin is affecting is not as root-cause-y as that which reprogramming targets.
Systemic rejuvenation is not enough
Even reprogramming doesn't do everything. I think no matter how much one reprograms one will eventually get cancer (cancer can overwhelm even a young immune system after all, see child leukemias!). Thus there are a series of things that are required for continued increases in healthy lifespan that are very targeted to specific ways to our biology: cancer therapies being next gen chemotherapies like 6-thio or immunotherapies (Like our own T-cell therapy!), amyloid plaque targeting therapies (if cellular rejvenation can't clear that), atherosclerosis targeting therapies like Repair Bio's, therapies to add new cells that were lost (Can't rejuvenate what's not there anymore!) and potentially more.
One last word on rapamycin-like drugs
Though I spend my time thinking about the future and comprehensive solutions to the aging problem, in the shorter term there are drugs that potentially one could take that could help one live longer healthier lives here and now. With its downsides and upsides, rapamycin might be worth using for some people in some situations, and more trials of this one molecule in humans are something I welcome.
Appendix: claims regarding rapamycin and aging in various sources
- Rapamycin slows aging in mice (2012, Wilkinson et al.)
- "Rapamycin slows aging in mice"
- "testing the idea that rapamycin might slow aging effects on many tissues and thus by inference slow the aging process per se"
- "the aging process is delayed by rapamycin in genetically heterogeneous mice."
- Rapamycin extends life and health span because it slows aging (2013, Blagosklonny)
- "Rapamycin extends life and healthspan because it slows aging"
- "what is aging if not an increase of the probability of death with age"
- Resveratrol and rapamycin: are they anti-aging drugs? (2021, Kaeberlein et al.)
- "A definitive link between TOR signaling and mammalian aging was established this summer in a report from the National Institute on Aging Interventions Testing Program in which the TOR inhibitor rapamycin was shown to increase life span in mice."
- Rapamycin: Prevention&Treatment of aging and age-related disease
- "Humans and other terrestrial mammals have programmed death by Aging. Anti-Aging medicine is about blocking pro-death/pro-aging pathways. The most robust pro-Aging is mTOR (mechanistic Target of Rapamycin). Rapamycin is the most effective drug to block mTOR. Hence anti-aging medicine is called Rapamycin medicine."
- Rapamycin rejuvenates oral health in aging mice (An et al., 2020)
- "The FDA-approved drug rapamycin slows aging and extends lifespan in multiple organisms, including mice"
Citation
In academic work, please cite this essay as:
Ricón, José Luis, “Rapamycin is not an aging drug. But what is an aging drug?”, Nintil (2023-12-10), available at https://nintil.com/rapamycin-not-aging/.